Consider the following data: 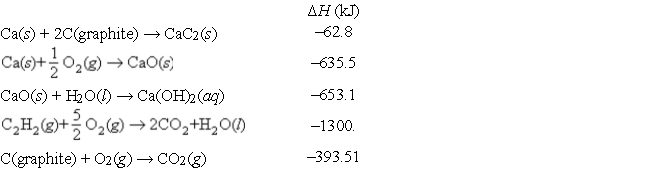
Use Hess's law to find the change in enthalpy at 25°C for the following equation:
CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH)2(aq)
Correct Answer:
Verified
Q58: At 25°C, the following heats of reaction
Q59: The enthalpy of fusion of ice is
Q60: Consider the following numbered processes:
1) A →
Q61: Consider the following reaction:
2Al(s) + 3Cl2(g) →
Q62: The heat combustion of acetylene, C2H2(g), at
Q64: Acetylene (C2H2) and butane (C4H10) are gaseous
Q65: The standard state of carbon as a
Q66: For which of the following reaction(s) is
Q67: The standard enthalpy change for the following
Q68: Which of the following statements is true
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents