Which of the following statements is true for a monatomic ideal gas?
A) Cv  Cp
Cp
B) Cv 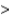 Cp
Cp
C) Cv = Cp + R
D) Cp = 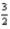 R
R
E) Cv is temperature dependent
Correct Answer:
Verified
Q63: Consider the following data: Q64: Acetylene (C2H2) and butane (C4H10) are gaseous Q65: The standard state of carbon as a Q66: For which of the following reaction(s) is Q67: The standard enthalpy change for the following Q69: Using the information below, calculate ΔH°f for Q70: Consider the following standard heats of formation: Q71: The heat of formation of Fe2O3(s) is Q72: The combustion of methanol takes place according Q73: Using the following data, calculate the standard![]()
P4O10(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents