Consider the reaction
2SO2(g) + O2(g) 
2SO3(g)
At constant temperature. Initially a container is filled with pure SO3(g) at a pressure of 2 atm, after which equilibrium is allowed to be reached. If y is the partial pressure of O2 at equilibrium, what is the value of Kp?
A) 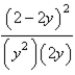
B) 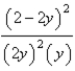
C) 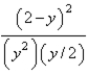
D) 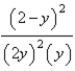
E) none of these
Correct Answer:
Verified
Q35: Nitric oxide, an important pollutant in air,
Q36: For a particular system at a particular
Q37: Consider the following equilibrium:
N2(g) + 3H2(g)
Q38: Consider the following equilibrium: Q39: Consider the equation 2A(g) Q41: Nitrogen gas (N2) reacts with hydrogen gas Q42: Explain how a given system at a Q43: Which of the following statements is false? Q44: Raising the pressure by lowering the volume Q45: Consider the following equilibrium: Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
A)![]()

