Based on the van der Waals equation of state 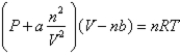 Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas. 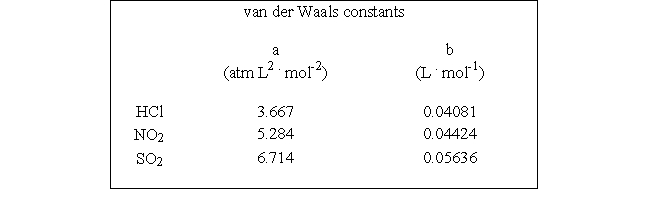
A) NO2
B) Must know P and V to answer this question
C) Must know n to answer this question
D) SO2
E) HCl
Correct Answer:
Verified
Q108: Near sea level, the atmosphere is composed
Q109: A certain element (Z) reacts to form
Q110: Determine the collision frequency for an oxygen
Q111: NOTES:general chemistry
-A scrubber removes the sulfur dioxide
Q112: With respect to the Maxwell-Boltzmann probability distribution
Q113: A gaseous mixture is 50% helium gas
Q114: Force per impact versus the molar mass
Q115: Suppose a sealed flask contains 1.0 L
Q116: Complete the following conversion:
1 atm = 760
Q117: Consider the decomposition of C5H6O3 as follows:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents