In this Lewis structure,which atom does NOT have a complete valence? 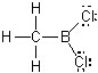
A) H
B) B
C) C
D) Cl
Correct Answer:
Verified
Q9: The Lewis structure of the cyanide ion
Q10: Which molecule does NOT have a lone
Q11: Which statement is CORRECT concerning the cyanide
Q12: A phosphorus atom with four bonds has
Q13: What is the formal charge on the
Q15: Which Lewis structure BEST describes the carbonate
Q16: Which compound has 24 valence electrons?
A) C3H6O
B)
Q17: A neutral carbon atom has _ valence
Q18: A oxygen atom with one bond and
Q19: A neutral sulfur atom has _ valence
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents