Which statement is CORRECT concerning the cyanide ion? 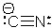
A) The carbon atom has a tetrahedral geometry,and the nitrogen atom has a liner geometry.
B) The carbon atom has a trigonal planar geometry,and the nitrogen atom has a liner geometry.
C) Both atoms have a linear geometry.
D) Both atoms have a trigonal planar geometry.
Correct Answer:
Verified
Q6: How many atoms in this molecule have
Q7: How many atoms in this molecule have
Q8: What is the electronic geometry around the
Q9: The Lewis structure of the cyanide ion
Q10: Which molecule does NOT have a lone
Q12: A phosphorus atom with four bonds has
Q13: What is the formal charge on the
Q14: In this Lewis structure,which atom does NOT
Q15: Which Lewis structure BEST describes the carbonate
Q16: Which compound has 24 valence electrons?
A) C3H6O
B)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents