Write the solubility product expression for the following solubility equilibrium: CuOH (s) Cu+ (aq) + OH- (aq)
A) 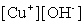
B) 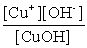
C) 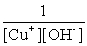
D) 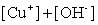
Correct Answer:
Verified
Q20: Which factor tends to increase the rate
Q21: Considering the solubility product values for the
Q22: The equilibrium expression of the following
Q23: If an acetic acid,HC2H3O2,buffer solution contains
Q24: Considering the solubility product values for the
Q26: Given the following equation and equilibrium
Q27: Consider the equilibrium reaction between the
Q28: Write the solubility product expression for
Q29: Which K value indicates a reaction that
Q30: The symbol used to represent the equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents