Write the solubility product expression for the following solubility equilibrium: BaSO4 (s) Ba2+ (aq) + SO42- (aq)
A) 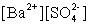
B) 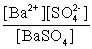
C) 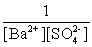
D) 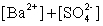
Correct Answer:
Verified
Q23: If an acetic acid,HC2H3O2,buffer solution contains
Q24: Considering the solubility product values for the
Q25: Write the solubility product expression for
Q26: Given the following equation and equilibrium
Q27: Consider the equilibrium reaction between the
Q29: Which K value indicates a reaction that
Q30: The symbol used to represent the equilibrium
Q31: In a saturated solution of aluminum phosphate,AlPO4,both
Q32: Consider the equilibrium reaction between the
Q33: In a saturated solution of cobalt(II)carbonate,CoCO3,both [Co2+]
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents