What is the half-reaction for the oxidation of sulfide ions in aqueous solution?
A) S2-(aq) 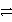 S(s) + 2 e-
S(s) + 2 e-
B) S-(aq) + e- 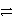 S2-(aq)
S2-(aq)
C) S(s) + 2 e- 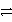 S2-(aq)
S2-(aq)
D) SO32-(aq) + 6 H+(aq) 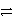 S(s) + 3 H2O(
S(s) + 3 H2O( 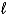 ) + 4 e-
) + 4 e-
E) SO42-(aq) + 8 H+(aq) 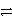 S(s) + 4 H2O(
S(s) + 4 H2O( 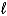 ) + 6 e-
) + 6 e-
Correct Answer:
Verified
Q2: What is the oxidation number of Cl
Q2: Consider the following images.The reactions occurring in
Q4: What is the balanced redox equation that
Q4: What happens in a galvanic cell?
A)Cations are
Q6: Identify the reduction half-reaction in the redox
Q9: Identify the oxidation half-reaction in the redox
Q9: What is the oxidation number of titanium
Q11: Which among the following are the parts
Q19: Oxidation-reduction reactions are also known as...
A)electron-transfer reactions
B)proton-transfer
Q20: Which of the following processes is a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents