Consider the following table of relative strengths of oxidizing and reducing agents:
Oxidizing Agent
Reducing Agent
Stronger
A+ + e- 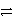
A
Weaker
B2+ + 2 e- 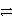
B
Weaker
C3+ + 3 e- 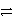
C
Stronger
Which correctly lists the oxidizing agents in order of increasing strength?
A) A < B < C
B) C < B < A
C) A+ < B2+ < C3+
D) B2+ < A+ < C3+
E) C3+ < B2+ < A+
Correct Answer:
Verified
Q24: What evidence suggests that Na+ ions are
Q32: What happens to an elemental oxidizing agent
Q33: Silver metal will not react with hydrochloric
Q35: What is the oxidation number of chromium
Q38: Which substance gets reduced in this redox
Q39: Balance the following redox reaction in acidic
Q41: Balance the half-reaction in acidic solution and
Q42: In balancing the half-reaction Q43: Balance the following redox equation in acidic Q44: In balancing the half-reaction
SO42-(aq) ![]()
NO(g) ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents