A hydrochloric acid solution is standardized by titrating against solid sodium carbonate as shown in the following image.The HCl is in the buret and the sodium carbonate in the flask.  The equation is 2 HCl(aq) + Na2CO3(s) 2 NaCl(aq) + H2O(
The equation is 2 HCl(aq) + Na2CO3(s) 2 NaCl(aq) + H2O( 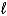 ) + CO2(g) .If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
) + CO2(g) .If 23.4 mL of the solution is added from the buret to neutralize 0.157 g Na2CO3 in the flask.what is the molarity of the HCl solution?
A) 0.0316 M
B) 0.0633 M
C) 7.90 M
D) 0.253 M
E) 0.127 M
Correct Answer:
Verified
Q20: Calculate the number of grams of ethyl
Q24: What is the equivalent mass of
Q25: What volume of hydrogen gas,measured at STP,is
Q26: Silver nitrate is commonly used to
Q28: Calculate the normality of a potassium
Q31: Write the balanced equation for the reaction
Q38: Calculate the normality of a solution made
Q40: How many equivalents of sulfuric acid will
Q44: Determine the decrease in the freezing point
Q45: Calculate the increase in boiling point of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents