Calculate the volume of hydrogen,measured at 19°C and 688 torr,that will be produced from 0.45 g of sodium in the reaction 2 Na(s) + 2 H2O( 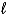 ) 2 NaOH(aq) + H2(g) .
) 2 NaOH(aq) + H2(g) .
A) 1.9 *102 mL
B) 2.1 * 102 mL
C) 2.3 * 102 mL
D) 2.6 * 102 mL
E) 1.0 *103 mL
Correct Answer:
Verified
Q25: If the molar volume of a gas
Q29: Calculate the volume of 76.2 mole of
Q29: What is the molar mass of a
Q30: How many liters of hydrogen,measured at
Q31: Calculate the molar volume of a gas
Q33: What is the molar volume of a
Q35: What is the volume of 0.0775 mole
Q36: What is the molar volume of CO2
Q37: Consider the reaction CH4(g)+ H2O(g)
Q39: Which of the following statements is incorrect?
i.The
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents