Consider the following periodic table. 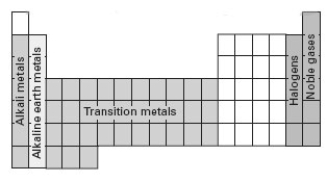 Which section represents elements whose electron configuration for the highest occupied energy level is ns2np5.
Which section represents elements whose electron configuration for the highest occupied energy level is ns2np5.
A) alkali metals
B) alkaline earth metals
C) transition metals
D) halogens
E) noble gases
Correct Answer:
Verified
Q27: Which of the following lists electron sublevels
Q28: What is the ground state electron configuration
Q30: Which of the following pairs of atomic
Q33: Identify the statement among the following that
Q34: In a given energy level,which of the
Q35: Which of the following elements has the
Q38: The maximum number of electrons that can
Q42: Which of the following is the best
Q43: How many of the following elements belong
Q43: Consider the following periodic table. 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents