Which of the following is the best definition of first ionization energy?
A) The energy required to remove one electron from a neutral gaseous atom of an element
B) The energy barrier that must be overcome to start a chemical reaction
C) The energy required to raise an electron in the ground state into the first excited state
D) The energy defined by 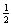 mv2
mv2
E) The energy needed to reduce a species in the aqueous phase to its minimum (first) reduction potential
Correct Answer:
Verified
Q27: Which of the following lists electron sublevels
Q30: Which of the following pairs of atomic
Q38: The maximum number of electrons that can
Q38: Consider the following periodic table. 
Q41: Which general electron configuration is responsible for
Q43: How many of the following elements belong
Q43: Consider the following periodic table. 
Q44: Which of the following is not a
Q46: Consider the following periodic table. 
Q46: What do elements in a family or
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents