Write the equilibrium expression for the following reaction: S(s) + O2(g)  SO2(g)
SO2(g)
A) K = 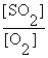
B) K = 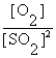
C) K = 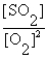
D) K = 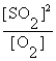
E) none of these
Correct Answer:
Verified
Q1: Choose the correct equilibrium expression for the
Q2: At equilibrium, the concentrations of all reactants
Q3: A catalyst is a substance that speeds
Q4: A minimum energy called the activation energy
Q6: At equilibrium, the concentrations of all reactants
Q7: Which of the following is an example
Q8: The correct equilibrium expression for the reaction
Q9: Write the equilibrium expression for the reaction
3O2(g)
Q10: For the reaction F2(g) Q11: The _ model explains why a reaction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents