Choose the correct equilibrium expression for the following reaction. A(s) + 2B(l) 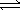 3C(aq) + 4D(aq)
3C(aq) + 4D(aq)
A) 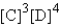
B) 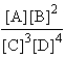
C) 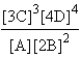
D) 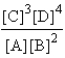
E) none of these
Correct Answer:
Verified
Q2: At equilibrium, the concentrations of all reactants
Q3: A catalyst is a substance that speeds
Q4: A minimum energy called the activation energy
Q5: Write the equilibrium expression for the following
Q6: At equilibrium, the concentrations of all reactants
Q7: Which of the following is an example
Q8: The correct equilibrium expression for the reaction
Q9: Write the equilibrium expression for the reaction
3O2(g)
Q10: For the reaction F2(g) Q11: The _ model explains why a reaction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents