Consider the following equilibrium: 2H2(g) + X2(g)  2H2X(g) + energy
2H2X(g) + energy
The equilibrium expression is
A) 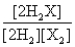
B) 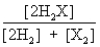
C) 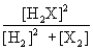
D) 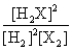
E) none of these
Correct Answer:
Verified
Q20: Consider the equilibrium shown here: CaCO3(s)
Q21: Consider the following equilibrium: 2H2(g) + X2(g)
Q22: Given the equation A(g) Q23: Consider the following equilibrium: H2(g) + I2(s) Q24: Given the equation A(g) Q26: Consider the following equilibrium: 2H2(g) + X2(g) Q27: Consider the reaction 2H2(g) + O2(g) Q28: The concentrations of pure solids or pure Q29: Given the equation A(g) Q30: Consider the reaction system CH4(g) + 2O2(g) Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

