Given the equation A(g) 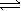 B(g) + 2C(g) . At a particular temperature, K =
B(g) + 2C(g) . At a particular temperature, K = 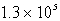 . If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
. If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
A) The reaction will proceed to the right.
B) The reaction will proceed to the left.
C) The mixture is at equilibrium.
D) More information is needed to answer the question.
Correct Answer:
Verified
Q19: Which of the following is an example
Q20: Consider the equilibrium shown here: CaCO3(s)
Q21: Consider the following equilibrium: 2H2(g) + X2(g)
Q22: Given the equation A(g) Q23: Consider the following equilibrium: H2(g) + I2(s) Q25: Consider the following equilibrium: 2H2(g) + X2(g) Q26: Consider the following equilibrium: 2H2(g) + X2(g) Q27: Consider the reaction 2H2(g) + O2(g) Q28: The concentrations of pure solids or pure Q29: Given the equation A(g) ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents