The solubility of Mg(OH) 2(s) in water at a certain temperature is 1.1 *10-4 mol/L. Calculate the value of Ksp for Mg(OH) 2(s) at this temperature.
A) 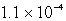
B) 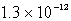
C) 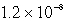
D) 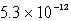
E) none of these
Correct Answer:
Verified
Q46: Lithium carbonate, Li2CO3 has a Ksp of
Q47: Write the balanced equation for the dissolving
Q48: Given the solubility products (Ksp) BaSO4
1.5* 10-9
CoS
5.0
Q49: The Ksp for ZnS(s) is 3.0 *10-22
Q50: The solubility in mol/L of Ag2CrO4 is
Q52: For the reaction 2SO2(g) + O2(g)
Q53: The solubility of Co(OH)2 in water at
Q54: For the reaction CO(g) + H2O(g)
Q55: The solubility of Cd(OH)2 in water at
Q56: The molar solubility of PbI2 at a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents