Lithium carbonate, Li2CO3 has a Ksp of 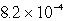 at a certain temperature. What is the solubility in mol/L for Li2CO3 at this temperature?
at a certain temperature. What is the solubility in mol/L for Li2CO3 at this temperature?
A) 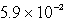
B) 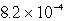
C) 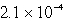
D) 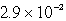
E) 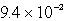
Correct Answer:
Verified
Q41: The solubility of BaCO3(s) in water at
Q42: Calculate the concentration of the copper ion
Q43: The solubility of ZnS(s) in water at
Q44: Write the balanced equation for the dissolving
Q45: Write the balanced equation for the dissolving
Q47: Write the balanced equation for the dissolving
Q48: Given the solubility products (Ksp) BaSO4
1.5* 10-9
CoS
5.0
Q49: The Ksp for ZnS(s) is 3.0 *10-22
Q50: The solubility in mol/L of Ag2CrO4 is
Q51: The solubility of Mg(OH)2(s) in water at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents