Multiple Choice
An excess of Al and 7.9 mol of Br2 are reacted according to the equation 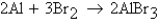 How many moles of AlBr3 will be formed assuming 100% yield?
How many moles of AlBr3 will be formed assuming 100% yield?
A) 2.6 mol
B) 4.0 mol
C) 5.3 mol
D) 7.9 mol
E) 11.9 mol
Correct Answer:
Verified
Related Questions
Q9: The equation Q10: Refer to the following unbalanced equation: Q11: Refer to the following equation: Q12: A 3.1-mol sample of KClO3 was decomposed![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents