How many molecules of carbon dioxide will be formed if 2.21 g of propane is burned in the following reaction? 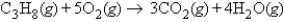
A) 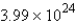 molecules
molecules
B) 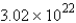 molecules
molecules
C) 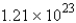 molecules
molecules
D) 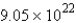 molecules
molecules
E) 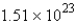 molecules
molecules
Correct Answer:
Verified
Q11: Refer to the following equation: 
Q12: A 3.1-mol sample of KClO3 was decomposed
Q13: The balanced equation Q14: An excess of Al and 7.9 mol Q15: A mole ratio is used to convert Q17: A balanced chemical equation is one that Q18: Which of the following reaction mixtures would Q19: Refer to the following equation: Q20: In the reaction Q21: Calculate the molecules of oxygen required to![]()

![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents