The rusting of iron is represented by the equation 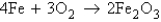 . If you have a 1.45-mol sample of iron, how many moles of Fe2O3 will there be after the iron has rusted completely?
. If you have a 1.45-mol sample of iron, how many moles of Fe2O3 will there be after the iron has rusted completely?
A) 0.483 mol
B) 0.725 mol
C) 0.97 mol
D) 1.45 mol
E) 2.18 mol
Correct Answer:
Verified
Q3: What mass of carbon dioxide will be
Q4: Refer to the following unbalanced equation:
Q5: Calculate the mass of water produced when
Q6: The balanced equation Q7: For the reaction Q9: The equation Q10: Refer to the following unbalanced equation: Q11: Refer to the following equation: Q12: A 3.1-mol sample of KClO3 was decomposed Q13: The balanced equation Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()

![]()

