Multiple Choice
Calculate the mass of water produced when 9.47 g of methane, CH4, reacts with an excess of oxygen in the following unbalanced reaction. 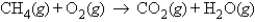
A) 10.64 g H2O
B) 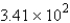 g H2O
g H2O
C) 21.3 g H2O
D) 0.526 g H2O
E) 1.18 g H2O
Correct Answer:
Verified
Related Questions
Q1: Consider the following reaction, where X represents
Q2: Refer to the following equation: 
Q3: What mass of carbon dioxide will be
Q4: Refer to the following unbalanced equation:
Q6: The balanced equation Q7: For the reaction Q8: The rusting of iron is represented by Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()

