Multiple Choice
In the reaction 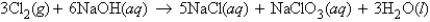 how many moles of chlorine molecules are needed to react with 13.3 g of NaOH?
how many moles of chlorine molecules are needed to react with 13.3 g of NaOH?
A) 1.00 mol Cl2
B) 0.333 mol Cl2
C) 0.67 mol Cl2
D) 0.166 mol Cl2
E) none of these
Correct Answer:
Verified
Related Questions
Q35: How many moles of O2 are required
Q36: How many moles of oxygen are produced
Q37: In the reaction Q38: For the reaction Q39: What number of moles of ammonia can![]()
![]()

