Multiple Choice
How many moles of oxygen are produced by decomposing 28.5 g of H2O2 (molar mass = 34.0 g/mol) according to the equation: 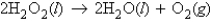
A) 0.838 mol O2
B) 485 mol O2
C) 0.419 mol O2
D) 1.68 mol O2
E) none of these
Correct Answer:
Verified
Related Questions
Q31: Calculate the mass of carbon dioxide produced
Q32: In the reaction Q33: For the reaction Q34: Methane, CH4, the major component of natural Q35: How many moles of O2 are required Q37: In the reaction Q38: For the reaction Q39: What number of moles of ammonia can Q40: In the reaction Q41: For the reaction Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()
![]()

