Multiple Choice
Calculate the mass of carbon dioxide produced from 22.3 g of octane, C8H18, in the following reaction. 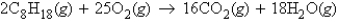
A) 68.7 g CO2
B) 137 g CO2
C) 77.3 g CO2
D) 1.074 g CO2
E) 1.562 g CO2
Correct Answer:
Verified
Related Questions
Q26: If 19.2 g of CO2 is produced
Q27: In the reaction Q28: Nitrogen and hydrogen gases are combined at![]()

