For the reaction 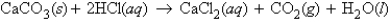 how many grams of CaCl2 can be obtained if 14.6 g HCl is allowed to react with excess CaCO3?
how many grams of CaCl2 can be obtained if 14.6 g HCl is allowed to react with excess CaCO3?
A) 44 g CaCl2
B) 89 g CaCl2
C) 0.200 g CaCl2
D) 22.2 g CaCl2
E) none of these
Correct Answer:
Verified
Q28: Nitrogen and hydrogen gases are combined at
Q29: For the reaction Q30: Consider the reaction Q31: Calculate the mass of carbon dioxide produced Q32: In the reaction Q34: Methane, CH4, the major component of natural Q35: How many moles of O2 are required Q36: How many moles of oxygen are produced Q37: In the reaction Q38: For the reaction Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()
![]()

