A sample with 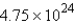 atoms of copper represents how many moles of copper?
atoms of copper represents how many moles of copper?
A) 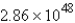 mol
mol
B) 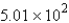 mol
mol
C) 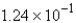 mol
mol
D) 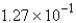 mol
mol
E) 7.89 mol
Correct Answer:
Verified
Q61: Consider separate 100.0-g samples of each of
Q62: Calculate the mass of 3.663 moles of
Q63: Calculate the percentage composition (by mass) of
Q64: Calculate the number of moles of Cl2
Q65: A hydrocarbon has the formula C5H12. What
Q67: Calculate the number of molecules in 0.000400
Q68: Calculate the number of molecules of CH4
Q69: How many molecules are present in
Q70: A 50.3-g sample of H2O contains how
Q71: Compound X2Y is known to be 60%
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents