Object A, with heat capacity CA and initially at temperature TA, is placed in thermal contact with object B, with heat capacity CB and initially at temperature TB.The combination is thermally isolated.If the heat capacities are independent of the temperature and no phase changes occur, the final temperature of both objects is:
A) (CATA - CBTB) /(CA + CB)
B) (CATA + CBTB) /(CA + CB)
C) (CATA - CBTB) /(CA - CB)
D) 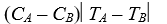
E) 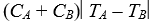
Correct Answer:
Verified
Q47: The formation of ice from water is
Q48: Two different samples have the same mass
Q49: Take the mechanical equivalent of heat as
Q50: An insulated container, filled with water, contains
Q51: The heat of fusion of water is
Q53: The heat capacity of object B is
Q54: The energy given off by 300 grams
Q55: Heat is:
A)energy transferred by virtue of a
Q56: Heat has the same units as:
A)temperature
B)work
C)energy/time
D)heat capacity
E)energy/volume
Q57: The heat capacity of an object is:
A)the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents