Pressure vs.volume graphs for a certain gas undergoing five different cyclic processes are shown below.During which cycle does the gas do the greatest positive work? 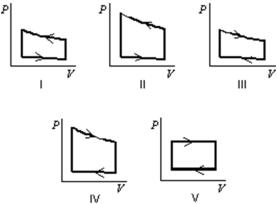
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q70: In a constant-volume process with a gas,
A)no
Q71: In the figure, what is the sign
Q72: According to the first law of thermodynamics,
Q73: A gas:
A)does positive work as it expands.
B)does
Q74: Fifty grams of ice at 0 °
Q76: In a certain process a gas ends
Q77: Of the following which might NOT be
Q78: Of the following which might NOT be
Q79: In the first law of thermodynamics, the
Q80: In an adiabatic process:
A)the energy absorbed as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents