In the figure, a gas undergoes a transition from point A to point B along the path shown.How much work is done by the gas? 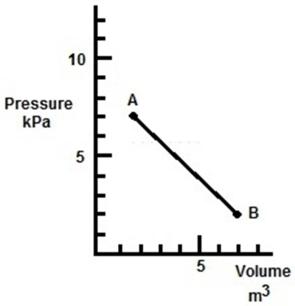
A) 10 kJ
B) 13 kJ
C) 23 kJ
D) -23 kJ
E) 0 kJ
Correct Answer:
Verified
Q60: A metal sample of mass M requires
Q61: In the first law of thermodynamics, the
Q62: How many calories are required to change
Q63: A certain humidifier operates by raising water
Q64: Steam at 100°C enters a radiator and
Q66: A system undergoes an adiabatic process in
Q67: Ten grams of ice at -20°C is
Q68: During an adiabatic process an object does
Q69: Solid A, with mass M, is at
Q70: In a constant-volume process with a gas,
A)no
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents