An ideal gas of N diatomic molecules has temperature T.If the number of molecules is doubled without changing the temperature, the internal energy increases by:
A) 0
B) 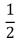 NkT
NkT
C) 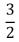 NkT
NkT
D) 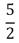 NkT
NkT
E) 3 NkT
Correct Answer:
Verified
Q86: Assume that helium behaves as an ideal
Q87: The number of degrees of freedom of
Q88: The number of degrees of freedom of
Q89: The specific heat of a polyatomic gas
Q90: A monatomic gas can have internal energy
Q92: The difference between the molar specific heat
Q93: For a given change in temperature, the
Q94: An ideal gas of N monatomic molecules
Q95: Assume that helium behaves as an ideal
Q96: An ideal gas has molar specific
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents