Compare the two reaction coordinate diagrams below and select the answer that CORRECTLY describes their relationship. In each case, the single intermediate is the ES complex. 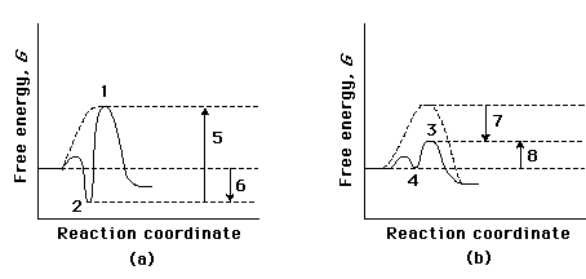
A) (a) describes a strict "lock and key" model, whereas (b) describes a transition-state complementarity model.
B) The activation energy for the catalyzed reaction is 5 in (a) and is 7 in (b) .
C) The activation energy for the uncatalyzed reaction is given by 5 + 6 in (a) and by 7 + 4 in (b) .
D) The contribution of binding energy is given by 5 in (a) and by 7 in (b) .
E) The ES complex is given by 2 in (a) and 3 in (b) .
Correct Answer:
Verified
Q8: Which statement is TRUE of the binding
Q9: Enzymes are potent catalysts because they:
A) are
Q10: Which enzymes are NOT among the six
Q11: An enzyme-catalyzed reaction was carried out
Q12: For enzymes in which the slowest (rate-limiting)
Q14: Michaelis and Menten assumed that the overall
Q15: Which statement about enzyme-catalyzed reactions is FALSE?
A)
Q16: In the following diagram of the first
Q17: Which statement is FALSE?
A) A reaction
Q18: The following data were obtained in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents