What is the mass of potassium hydrogen carbonate (100.12 g/mol) that decomposes to release 4.25 L of carbon dioxide gas at STP? __KHCO₃(s) 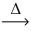 __k₂CO₃(s) + __H₂O(l) + __CO₂(g)
__k₂CO₃(s) + __H₂O(l) + __CO₂(g)
A) 0.476 g
B) 1.90 g
C) 9.50 g
D) 19.0 g
E) 38.0 g
Correct Answer:
Verified
Q76: What is the mass of insoluble lead(II)iodide
Q77: What is the mass of sodium nitrate
Q78: What is the mass of aluminum oxide
Q79: What is the mass of hydrogen gas
Q80: What is the volume of oxygen gas
Q82: What volume of oxygen gas reacts with
Q83: What volume of hydrogen gas reacts to
Q84: What volume of oxygen gas reacts with
Q85: What volume of oxygen gas reacts to
Q86: What is the volume of carbon dioxide
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents