What volume of oxygen gas reacts to produce 20.0 mL of chlorine gas? (Assume temperature and pressure remain constant. )
__ HCl(g) + __O₂(g) 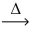 __Cl₂(g) + __H₂O(g)
__Cl₂(g) + __H₂O(g)
A) 5.00 mL
B) 10.0 mL
C) 20.0 mL
D) 40.0 mL
E) none of the above
Correct Answer:
Verified
Q80: What is the volume of oxygen gas
Q81: What is the mass of potassium hydrogen
Q82: What volume of oxygen gas reacts with
Q83: What volume of hydrogen gas reacts to
Q84: What volume of oxygen gas reacts with
Q86: What is the volume of carbon dioxide
Q87: What volume of propane gas,C₃H₈,reacts to give
Q88: What volume of hydrogen gas reacts with
Q89: What is the volume of hydrogen gas
Q90: What is the mass of bismuth carbonate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents