Considering the limiting reactant,what is the mass of manganese produced from 25.0 g of manganese(IV) oxide (86.94 g/mol) and 25.0 g of aluminum metal? 3 MnO₂(l) + 4 Al(l) 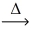 3 Mn(l) + 2 Al₂O₃(s)
3 Mn(l) + 2 Al₂O₃(s)
A) 15.8 g
B) 38.2 g
C) 47.4 g
D) 50.9 g
E) 67.8 g
Correct Answer:
Verified
Q113: Considering the limiting reactant,what is the mass
Q114: Considering the limiting reactant concept,how many moles
Q115: Considering the limiting reactant concept,how many moles
Q116: Considering the limiting reactant,what is the volume
Q117: Considering the limiting reactant,what is the volume
Q119: Starting with 1.550 g of potassium chlorate,a
Q120: Considering the limiting reactant,what is the volume
Q121: The decomposition of 1.500 g of baking
Q122: Considering the limiting reactant,how many moles of
Q123: How many moles of oxygen gas react
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents