Considering the limiting reactant,what is the volume of NO gas produced from 40.0 L of ammonia gas and 40.0 L of oxygen gas? (Assume constant conditions. ) 4 NH₃(g) + 5 O₂(g) 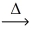 4 NO(g) + 6 H₂O(g)
4 NO(g) + 6 H₂O(g)
A) 32.0 L
B) 40.0 L
C) 50.0 L
D) 80.0 L
E) none of the above
Correct Answer:
Verified
Q115: Considering the limiting reactant concept,how many moles
Q116: Considering the limiting reactant,what is the volume
Q117: Considering the limiting reactant,what is the volume
Q118: Considering the limiting reactant,what is the mass
Q119: Starting with 1.550 g of potassium chlorate,a
Q121: The decomposition of 1.500 g of baking
Q122: Considering the limiting reactant,how many moles of
Q123: How many moles of oxygen gas react
Q124: How many moles of water are produced
Q125: How many molar masses of HCl gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents