How many molar masses of CO₂ gas are produced from 2 molar masses (MM) of carbon monoxide gas according to the following equation? __CO(g) + __O₂(g) 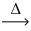 __CO₂(g)
__CO₂(g)
A) 1 MM
B) 2 MM
C) 3 MM
D) 4 MM
E) none of the above
Correct Answer:
Verified
Q123: How many moles of oxygen gas react
Q124: How many moles of water are produced
Q125: How many molar masses of HCl gas
Q126: The reaction for photosynthesis producing glucose sugar
Q127: The reaction for photosynthesis producing glucose sugar
Q129: What volume of carbon monoxide gas at
Q130: The decomposition of 1.500 g of sodium
Q131: How many moles of carbon dioxide are
Q132: What law states the total volume of
Q133: How many moles of oxygen gas react
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents