If 1.65 g of nitrogen gas reacts completely with excess hydrogen gas,what is the volume of ammonia produced at 500.0 K and 500.0 atm? N₂(g) + H₂(g) 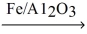 NH₃(g)
NH₃(g)
A) 0.00288 L
B) 0.00967 L
C) 1.32 L
D) 5.28 L
E) 2417 L
Correct Answer:
Verified
Q86: A sample of sodium carbonate is treated
Q87: What is the mass of nitrogen dioxide
Q88: Which of the following is a general
Q89: Which of the following problem-solving techniques is
Q90: If 10.0 L of nitrogen gas at
Q92: If a 0.079-g sample of unknown metal
Q93: Given the reaction for manufacturing hydrazine,N₂H₄,which in
Q94: What is the molar mass of an
Q95: How many water molecules are in a
Q96: If 100.0 mL of 0.150 M sodium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents