If 10.0 L of nitrogen gas at STP reacts completely with excess hydrogen gas,what is the volume of ammonia produced at 500.0 K and 500.0 atm? N₂(g) + H₂(g) 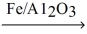 NH₃(g)
NH₃(g)
A) 0.0218 L
B) 0.0366 L
C) 0.0733 L
D) 10.9 L
E) 20.0 L
Correct Answer:
Verified
Q85: What is the mass of a 1.00-inch
Q86: A sample of sodium carbonate is treated
Q87: What is the mass of nitrogen dioxide
Q88: Which of the following is a general
Q89: Which of the following problem-solving techniques is
Q91: If 1.65 g of nitrogen gas reacts
Q92: If a 0.079-g sample of unknown metal
Q93: Given the reaction for manufacturing hydrazine,N₂H₄,which in
Q94: What is the molar mass of an
Q95: How many water molecules are in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents