For the exothermic reaction 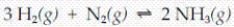 which of the following changes could be carried out in such a way as to not cause the reaction to shift either to the left or to the right?
which of the following changes could be carried out in such a way as to not cause the reaction to shift either to the left or to the right?
A) raising the temperature and adding NH3
B) lowering the temperature and removing NH3
C) both A) and b
D) neither a nor b
Correct Answer:
Verified
Q83: Consider the following energy diagram for a
Q84: A particular reaction has an equilibrium constant
Q85: When considering the effect of a catalyst
Q86: If the endothermic reaction Q87: Consider the following energy diagram for a Q89: Le Châtelier's Principle applies to which of Q90: Consider the following energy diagram for a Q91: A particular reaction has an equilibrium constant Q92: What is the effect of adding C2H5OH(l) Q93: Which of the following describes the effect![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents