Consider the following energy diagram for a reaction. 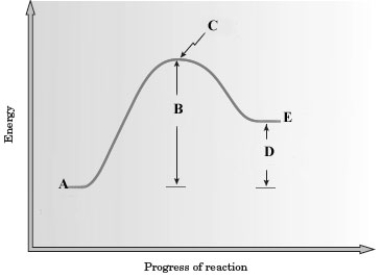
-Which letter represents the transition state?
A) A
B) B
C) C
D) D
E) E
Correct Answer:
Verified
Q85: When considering the effect of a catalyst
Q86: If the endothermic reaction Q87: Consider the following energy diagram for a Q88: For the exothermic reaction Q89: Le Châtelier's Principle applies to which of Q91: A particular reaction has an equilibrium constant Q92: What is the effect of adding C2H5OH(l) Q93: Which of the following describes the effect Q94: For the exothermic reaction Q95: If the exothermic reaction Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()

