Write an expression for the equilibrium constant (Keq) for the following reaction.  (g)
(g) 
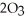 (g)
(g)
Correct Answer:
Verified
Q95: The term "molar solubility of a salt"
Q96: Predict the shift in the direction of
Q97: Predict the direction in which the reaction
Q98: Predict the shift in the direction of
Q99: Write an expression for the equilibrium constant
Q101: Identify each of the equilibrium constant values
Q102: For the following exothermic reaction:
CO +
Q103: Identify each of the equilibrium constant values
Q104: Identify each of the equilibrium constant values
Q105: For the following endothermic reaction:
C (s) +
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents