For the following exothermic reaction:
CO + 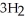

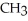 OH
OH
predict the direction of the reaction if:
a) temperature is decreased with the concentrations remaining the same.
b) a catalyst is added with the concentrations AND temperature remaining the same.
c) the concentration of 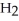 is halved.
is halved.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q97: Predict the direction in which the reaction
Q98: Predict the shift in the direction of
Q99: Write an expression for the equilibrium constant
Q100: Write an expression for the equilibrium constant
Q101: Identify each of the equilibrium constant values
Q103: Identify each of the equilibrium constant values
Q104: Identify each of the equilibrium constant values
Q105: For the following endothermic reaction:
C (s) +
Q106: Identify each of the equilibrium constant values
Q107: Predict the effect of increasing pressure in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents