Which is the correct equilibrium constant expression for the following reaction? 2C6H6(g) + 15O2(g)  12CO2(g) + 6H2O(g)
12CO2(g) + 6H2O(g)
A) 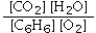
B) 
C) 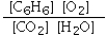
D) 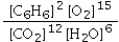
E) 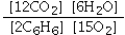
Correct Answer:
Verified
Q1: Which substances are included in the equilibrium
Q5: At elevated temperatures,hydrogen iodide may decompose to
Q7: The equilibrium constant for the reaction Ni(s)+
Q7: The observation that at equilibrium, the reaction
Q9: What is defined as a fraction with
Q10: Hydrogen peroxide may decompose to form water
Q11: Carbon tetrachloride reacts at high temperatures with
Q14: What is defined as a fraction with
Q17: During a chemical reaction, what defines when
Q18: Which statement is correct?
A) If Q <
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents