Consider reactions A,B,and C,which have the potential energy profiles shown.Assuming that the reactions have roughly the same frequency factors,which reaction is the slowest? 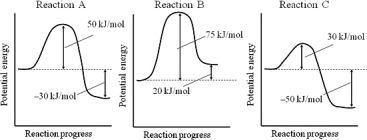
A) Reaction A
B) Reaction B
C) Reaction C
D) All of the reactions have the same rate.
Correct Answer:
Verified
Q66: Below is a plot of the reaction
Q67: The following diagram represents the zeroth-order decomposition
Q69: Carbon-14 is a radioactive isotope which decays
Q70: A rate constant obeys the Arrhenius equation,the
Q72: Consider the following potential energy profile for
Q74: Consider the following potential energy profile for
Q78: A reactant R is being consumed in
Q85: What is the name given to the
Q96: The isomerization of cyclopropane follows first-order kinetics.
Q98: What is the molecularity of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents