Below is a plot of the reaction of A decomposing to form products. 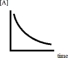 Based on the figure,which might be the order of the reaction with respect to A?
Based on the figure,which might be the order of the reaction with respect to A?
A) The reaction must only be zeroth order in A.
B) The reaction must only be first order in A.
C) The reaction must only be second order in A.
D) The reaction could be either first order in A or second order in A.
E) The reaction could be of any order in A.
Correct Answer:
Verified
Q29: The reaction CH3NC(g) → CH3CN(g) is first-order
Q61: kJ/mol. Sn2+ + 2Co3+ → Sn4+ +
Q62: The radioactive isotope tritium decays with a
Q67: The following diagram represents the zeroth-order decomposition
Q69: Carbon-14 is a radioactive isotope which decays
Q70: A rate constant obeys the Arrhenius equation,the
Q71: Consider reactions A,B,and C,which have the potential
Q87: The rate law for the reaction H2O2
Q94: The activation energy for the following first-order
Q96: The isomerization of cyclopropane follows first-order kinetics.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents