The following diagram represents the first-order decomposition of A to form X according to the following balanced chemical equation: A → X.Each sphere represents 1.0 mmol of atoms,and the volume of the box is 1.0 L. 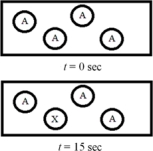 What is the half-life of the reaction?
What is the half-life of the reaction?
A) 15 s
B) 30 s
C) 36 s
D) 45 s
E) 60 s
Correct Answer:
Verified
Q17: The rate of a reaction is determined
Q91: Which is true concerning the following potential
Q93: The following diagram represents the second-order decomposition
Q95: The following is an Arrhenius plot of
Q97: The following is an Arrhenius plot of
Q102: A _-_ is a reaction whose rate
Q107: The intermediate in a reaction appears in
Q108: _ describes the reaction rate as being
Q109: The _ is the equation relating the
Q120: The rate law predicted by the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents