The following is an Arrhenius plot of a first-order reaction.The rate constant is measured in units of s-1. 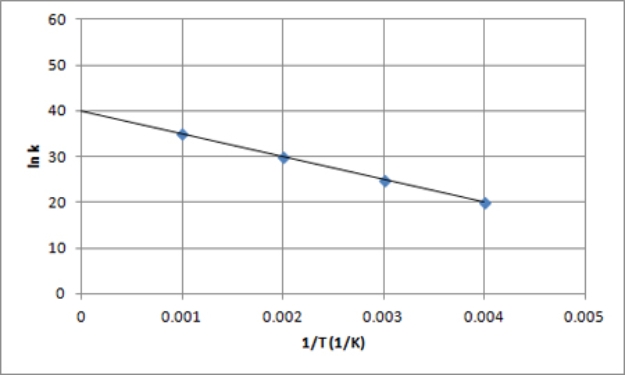 If a catalyst is added to the reaction,which could correspond to an Arrhenius plot of the catalyzed reaction?
If a catalyst is added to the reaction,which could correspond to an Arrhenius plot of the catalyzed reaction?
A) 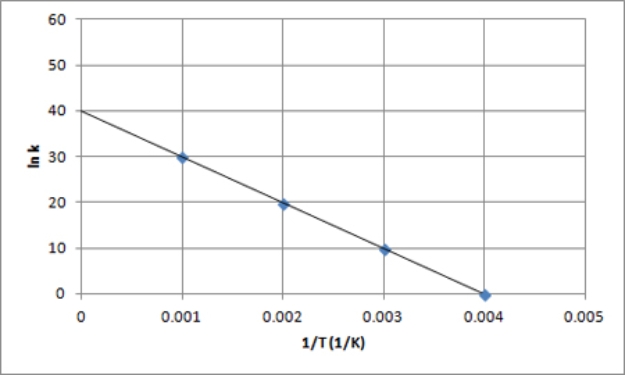
B) 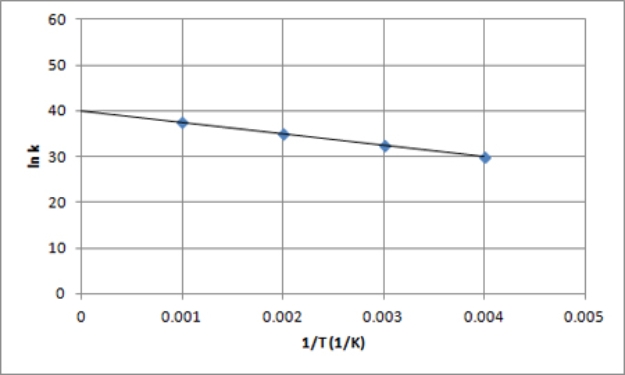
C) 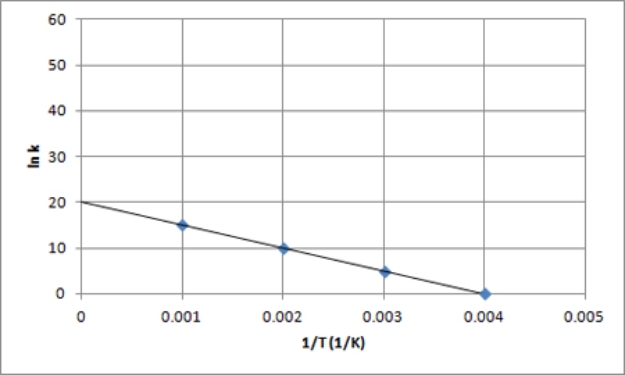
D) 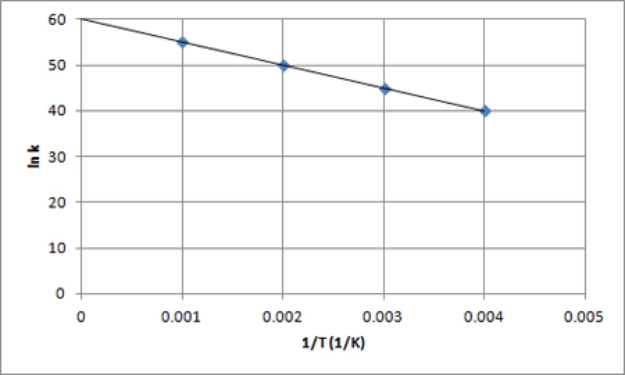
E) 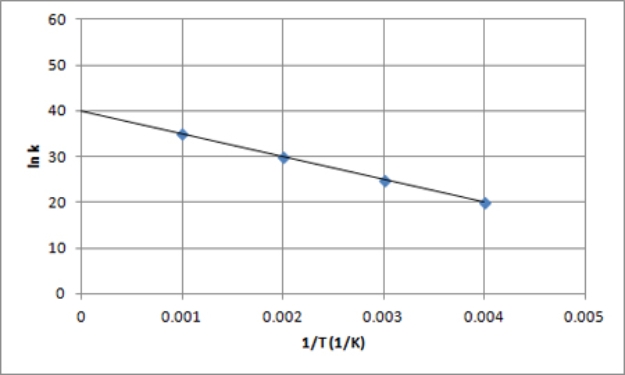
Correct Answer:
Verified
Q17: The rate of a reaction is determined
Q73: Is a bimolecular reaction necessarily second-order? Is
Q93: The following diagram represents the second-order decomposition
Q95: The following is an Arrhenius plot of
Q96: The following diagram represents the first-order decomposition
Q102: A _-_ is a reaction whose rate
Q107: The intermediate in a reaction appears in
Q108: _ describes the reaction rate as being
Q109: The _ is the equation relating the
Q120: The rate law predicted by the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents